STUDYING IONIC SURFACTANT MICELLES BY SMALL-ANGLE X-RAY AND NEUTRON SCATTERING
Project financed by the National Foundation for Scientific Research (OTKA), contract number T016184 and T029958
Sz. Vass, KFKI Atomic Energy Research Institute, Budapest, Hungary
S. Borbély, Research Institute for Solid State Physics and Optics, Budapest, Hungary
T. Gilányi, Department of Colloid Chemistry, Loránd Eötvös University, Budapest,
Hungary
P. Laggner, Institute for Biophysics and X-ray Structure Research, Graz, Austria
J. Pletíl, Institute of Macromolecular Chemistry, Prague, Czech Republic
Ionic surfactants are built up of (water-insoluble, hydrophobic) hydrocarbon chains and (water-soluble, hydrophilic) ionic headgropus. Due to the opposite tendencies in the solubility of these molecular segments, ionic surfactants in aqueous solution aggregate into micelles (without forming chemical bonds), each consisting of characteristically 40-100 monomers. The chains form a microscopic hydrocarbon droplet (of 2-3 nm radius) called micellar core such that the headgroups are located in the hydrocarbon/water interface where they dissociate into headgroup- and counter-ions, the latter being distributed in the aqueous pseudophase. The repetition of ionic aqueous-, and non-ionic hydrocarbon pseudophases in close vicinity results in interesting, structure-related, properties from various aspects of sciences and applications.
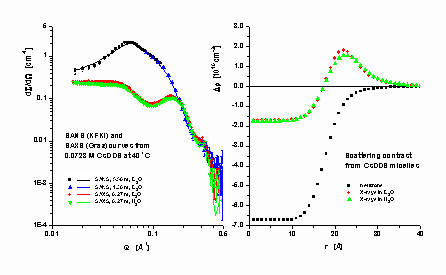
Direct information on the structure of micelles is obtained from the application of scattering methods. Nowadays the most suitable tool is the small-angle neutron- and X-ray scattering. In scattering experiments the basic concept is the scattering contrast, a quantity related to the spatial distribution of matter in the scatterer. The interpretation of scattering experiments is equivalent to elaborate models for this distribution. The most frequently used approach to the micellar structure assumes that the micelles are spheres or ellipsoids, which are divided into two confocal regions (two-shell model): the inner region stands for the core, the outer for all the other components.
Though this very crude approximation may lead to valuable knowledge on the size and shape of the micellar core and on the intermicellar interactions [1], the two-sell model is unable to reflect the fine details of the ionic distributions. Instead of the particle, in a new approach [2] we focus on its components, the micelle-forming molecules, which were di- vided into characteristic groups j and a probability density function j j(r) of finding them in location r is defined for each group. For example, in case of alkali alkyl sulphates the -CH3, -CH2-, -SO4- and Me+ groups have been arbitrarily - selected. The scattering amplitude A(Q) from a micelle equals S j (bj - vjr w)ò j j(r)exp[-iQr]dr, the linear combination of the Fourier-transforms of j j(r), where bj and vj is the scattering length and volume of group j, respectively, and r w is the scattering length density of the solvent. When incompressibility of liquids is assumed, vwj w(r)+S j vjj j(r) = 1, where subscript w stands for the solvent. This relationship makes possible to gather information from scattering on the spatial distribution of solvent molecules inside- and around the micelles, as well as on all colligative quantities related to the micelles. The above outlined, multicomponent, model is more suitable for characterizing the continuous changes in the micellar structure and in the distribution of the scattering contrast over the micelles than the previously used two-shell models.
Because the information content of small-angle scattering patterns is poor, both neutron (SANS) and X-ray (SAXS) patterns were recorded from the same system (under the same thermodynamic conditions), and were simultaneously fitted [3] by the multicom- ponent model. Difference in SANS and SAXS patterns is caused only by the difference in the contrast D r j = (bj - vj× r w) of the molecular groups. Consequently, simultaneous analysis is expected to result in more reliable knowledge of their structure and hydration properties. Results from H2O and D2O solutions of caesium dodecyl sulphate are seen in the figure, demonstrating that small differences in hydration like solvent isotope effects on the aggre- gation can be interpreted in terms of the multicomponent model along with reasonable structural parameters.

[1] Sz. Vass, T. Gilányi, S. Borbély, J. Phys. Chem. B104, 2073 (2000).
[2] Sz. Vass, J. Phys. Chem. B105, 455 (2001).
[3] Sz. Vass, J. Pletil, P. Laggner, S. Borbély, H. Pospíil, T. Gilányi,
Physica B, 276-278, 406 (2000)